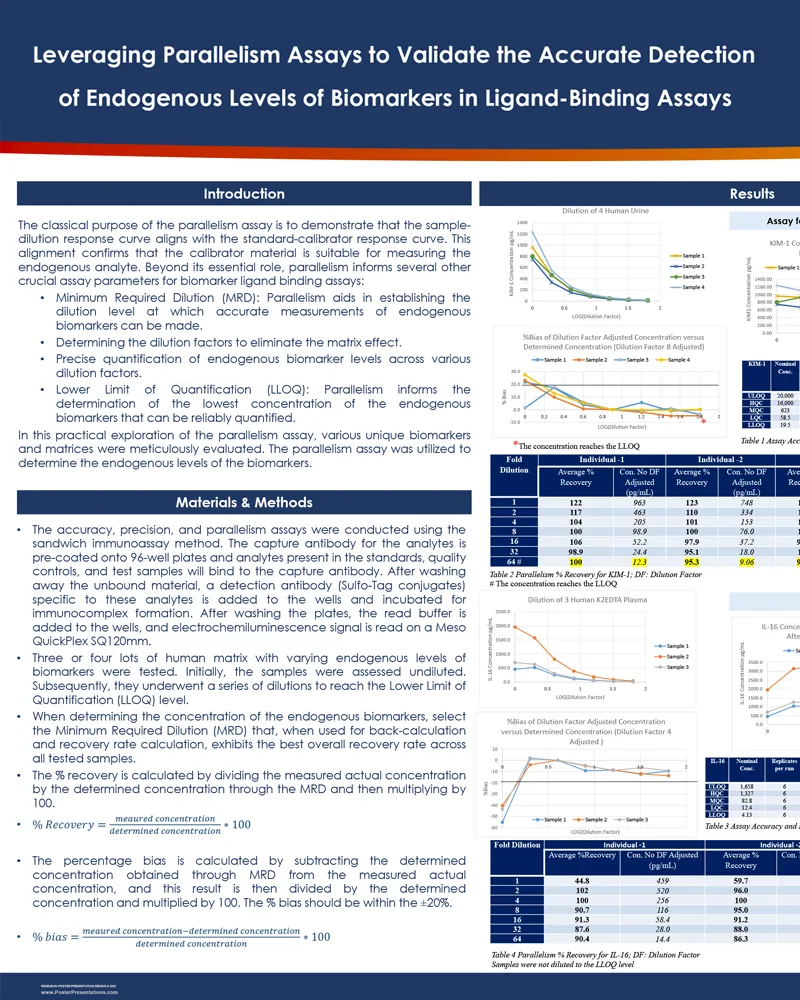
The parallelism assay is pivotal for ensuring that the sample-dilution response curve is aligned with the standard-calibrator response curve, confirming the suitability of the calibrator for measuring endogenous analytes. This alignment is critical not only for validating the calibrator but also aids in several key assay parameters for biomarker ligand binding assays:
- Minimum Required Dilution (MRD): It helps establish the necessary dilution level for accurate measurement of biomarkers.
- Matrix Effects: It assists in identifying dilution factors that mitigate matrix effects.
- Quantification: It enables precise quantification of biomarker levels across different dilution factors.
- Lower Limit of Quantification (LLOQ): It informs the determination of the lowest measurable biomarker concentration.
In a detailed study, various biomarkers and matrices were rigorously tested using the parallelism assay to measure endogenous biomarker levels.
For a deeper understanding and further details, download the comprehensive poster presented at this year’s WRIB conference.