
When conducting first-in-human (FIH) clinical trials, small to midsize pharmaceutical and biotechnology companies are faced with several crucial choices that can shape the trajectory of their drug development pathway. One key decision is whether to file an Investigational New Drug (IND) application with the US Food and Drug Administration (FDA) or a Clinical Trial Application (CTA) with the European Medicines Agency (EMA) and respective regulatory agencies of its member states.
While the nonclinical data required for both applications are fairly similar, it’s unrealistic for most companies to follow both paths, as the submission processes are complex and time-consuming. The two regulatory frameworks offer distinct benefits and disadvantages, but which one is right for your drug development program? Here are a few factors to consider:
Key Distinctions Between CTAs and INDs
One primary distinction to note between a CTA and an IND submission is that the CTA is protocol-specific, while the IND is product-specific. Once the CTA is submitted, the EU requires a new CTA for any new protocols amended. In contrast, once the IND submission is cleared, the sponsor may amend the filing with updates in order to add further protocols.
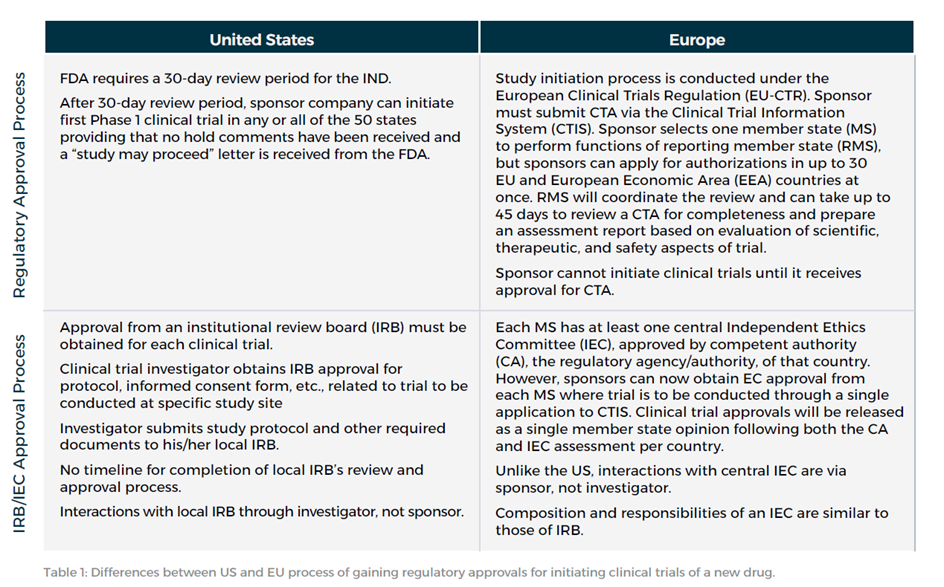
Check out the table below to understand the key differences between the US and EU processes for gaining regulatory approvals, with an emphasis on the distinct timelines and procedures associated with IND and CTA submissions:
Financial Considerations
When choosing between a CTA or IND application, your financial situation will likely play a considerable role in the decision-making process. Some questions to think about:
- What will it cost to complete FIH trials within the EU under the guidelines of a CTA compared to the cost of running trials under the guidelines of an IND in the US?
- Are there differential financial incentives to encourage sponsors to initiate early phase clinical research under a CTA, and how do those incentives compare to incentives that exist to initiate the same research under an IND?
In fact, multiple countries within the EU offer incentives to those programs operating under a CTA. For example, the Netherlands provides tax incentives, research and development credits, and more to clinical programs in their country. These incentives may make one pathway significantly more attractive than the other, particularly for small to midsize companies with limited funding.
The Utility of Reciprocity
Since all clinical trials must be performed in accordance with established Good Clinical Practice (GCP) guidelines, reciprocity between the FDA and EMA allows data from trials conducted under a CTA to be admissible for FDA submissions. Paired with considerable cost advantages, the CTA pathway may be more attractive to drug developers, as they have the opportunity to conduct trials in regions with lower operational costs.
Geographical Considerations
However, financial considerations may not be the only factor at play, as it’s also critical to contemplate geographical factors, including:
- Patient accrual rates
- Availability of subject matter experts
- Regional density of clinical trials
- Site and corporate relationships within the region
- Local standards of care
- Desired market country
Early Engagement with Regulatory Authorities
One method to “de-risk” the decision process when considering between an IND and CTA submission is to engage early and consistently with regulatory authorities. This engagement helps bolster the support of the payer/provider community and provides invaluable feedback on what should be included within the development program, enhancing the value of the asset.
Get Help with Your Decision with Worldwide
The decision between IND and CTA pathways for FIH studies is multifaceted, involving financial considerations, regional incentives, and early engagement with regulatory authorities. Understanding the nuances of each pathway is crucial for small to midsize companies aiming to navigate the complexities of global clinical development successfully.
For additional insights, download our full white paper, “First-in-Human Studies: IND or CTA?”.


