
With cost and timelines being two of the most significant factors influencing a study’s progression aside from efficacy and safety, master protocols offer an attractive option for study design. These designs facilitate streamlined trial logistics and centralized governance and create higher-quality data. But how do they work, and when are they appropriate?
What Are Master Protocols?
Master protocols allow a trial to perform multiple tests on diverse patient populations or diseases under a unified design. Basket and umbrella designs are the most commonly utilized types of master protocols. Selection of the appropriate design requires holistic consideration of your investigational product(s), patient population(s), and program specifics.
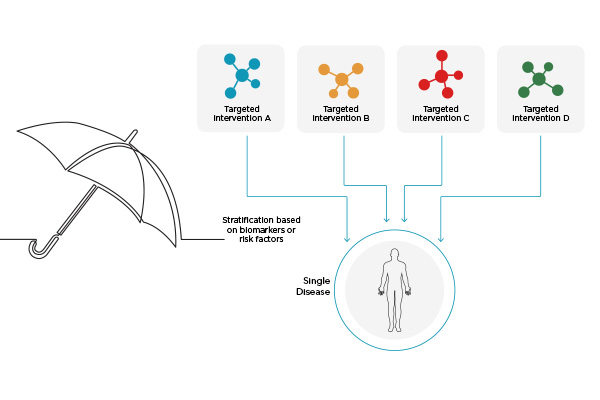
Umbrella Studies
Umbrella studies evaluate multiple investigational products (IPs) within a single disease. For example, an umbrella study could investigate different therapeutic agents to treat COVID-19 for hospitalized patients.
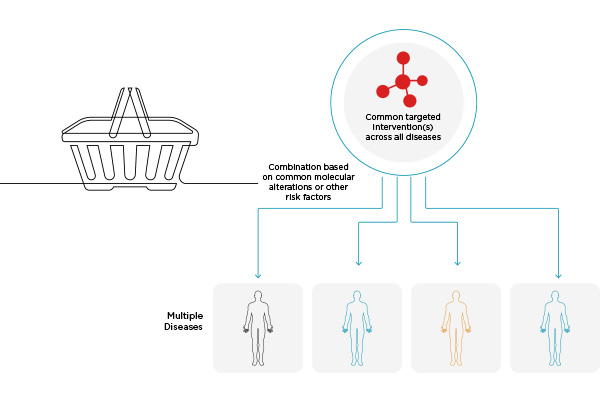
Basket Studies
Basket studies investigate a potential IP for efficacy across multiple conditions. For instance, many mechanisms in cancers are similar across different diagnoses, suggesting that some therapeutics may have a broader efficacy than in just one specific cancer type.
Both protocol designs utilize shared design and operational aspects, answering a greater number of questions more quickly and cost-effectively than individual trials would allow.
When Are Master Protocols Appropriate?
Choosing to use a master protocol will depend highly on your program’s specifics, such as:
- Do you have existing data for multiple products that suggest they could be efficacious for a given indication?
- Are there multiple viable indications to evaluate for your IP?
- Do you have various stakeholders invested in the success of your development programs?
Master protocols are frequently used in oncology trial designs and are expanding to other indications, including schizophrenia, Chron’s disease, Alzheimer’s disease, and pediatric Gaucher disease. Master protocols are also a viable option in rare disease trials because they help navigate small patient populations and can reduce operational costs.
To help determine if a master protocol could be a good fit for your study, check out this white paper for a more comprehensive overview of the appropriateness of master protocols in different contexts. Here are some relevant factors below:
What Are the Advantages of a Master Protocol?
Master protocols can allow drug developers to:
- Investigate multiple treatments related to a single disease
- Investigate a single treatment across multiple diseases
- Potentially reduce program costs
- Possibly reduce sample size
- Have the flexibility to modify group sizes based on real-time data trends, including adding necessary or removing extraneous groups
- Gather higher-quality data through unified data collection and sharing systems
Umbrella studies have the added benefit of being able to share a control group and can be easily adapted to include/remove branches as new therapeutic targets are discovered or data shows a branch to be ineffective. Basket studies, similarly, can be expanded to include new populations as needed, and they facilitate proof-of-concept study design.
Challenges to Consider
Despite their advantages, there are challenges associated with umbrella and basket study designs. Umbrella trials face statistical challenges like choosing between frequentist-based and Bayesian frameworks and controlling for error rates. Patient recruitment may also be difficult and require a strategic approach when distinct subgroups exist within a shared indication. Basket trials, on the other hand, may need a larger sample size than single trial protocols to detect effects accurately. For both designs, balancing multiple stakeholders with varying interests and priorities can create further complications while navigating your trials.
For a more in-depth look at the advantages and disadvantages of each, including potential ethical concerns and enrollment requirements, be sure to check out Master Protocols: The Opportunities and Challenges of Baskets and Umbrellas.
Master Protocols Are Useful but Not One-Size-Fits-All
Master protocols provide drug developers with opportunities to increase the efficiency and flexibility of their trials. Implementing a master protocol can be beneficial for certain investigations, but it’s important to have a thorough understanding of the associated challenges before deciding. Make a confident and informed decision about choosing a master protocol for your next trial with the help of the information published in this white paper.
If you have questions about whether a master protocol is right for your study or want more information, our drug development team is ready to talk with you. Contact us.


