
Every step in a successful oncology study requires critical and carefully thought-out decisions. Biomarkers provide a rapid way to inform many clinical oncology study development decisions, including study endpoints. What are they, how do they work, and how can they benefit your oncology study?
What Are Biomarkers, and How Are They Used in Oncology?
Biomarkers are measurable substances in a body, such as the presence of a given cell type or a change in a molecular ratio. They can inform intervention outcomes, serve as study endpoints, and are advantageous across the spectrum of cancer care. Currently, there are thousands of known biomarkers. From their use in risk and safety assessments to patient screening and treatment efficacy, biomarkers offer minimally invasive, objective data. Below, we discuss some validated circulating oncology biomarkers, their implementation, and key operational considerations. For a more in-depth look at biomarkers, check out our white paper.
Circulating Biomarkers Offer a Minimally Invasive Option for Oncology Study Endpoints
Healthcare professionals can draw circulating biomarkers from blood, plasma, or serum. Fortunately, this process is minimally invasive and easily attained, positioning circulating biomarkers to benefit studies requiring standardized, reproducible, and efficient results. Among the various biomarker categories, each has several beneficial characteristics for specific contexts. Some categories include:
- Non-specific markers of disease burden
- Tumor markers
- Circulating tumor cells
- Immuno-oncology (IO)
Non-Specific Markers of Disease Burden Indicate Damage or Dividing Cancer Cells
Non-specific markers of disease burden are biomarkers occurring as macromolecules that enter the bloodstream in one of two ways:
- Loss of cell membrane integrity
- Overexpression from rapidly dividing cancer cells
Biomarkers within this category are not cancer-specific, but they have been extensively studied in cancer and are well-understood in today’s medicine. Many of these markers have established baseline values to compare against your study’s data. Deviations from established baseline values provide insights into treatment response in certain cancer indications. In addition to being well-understood, the methods for collecting non-specific markers of disease burden are frequently cost-effective and robust, enabling your clinical program to collect data seamlessly.
Lactate dehydrogenase is one type of non-specific marker associated with cell damage across various indications, including myopathies, hemolytic anemias, and different cancers.
Tumor Markers Provide Valuable Insights into Clinical Care
In comparison to non-specific markers, tumor markers can provide more specific insights into tumor burden and treatment response to your compound. Some common tumor markers include:
- Cancer antigen 125: a well-known tumor marker with utility in response assessment, particularly ovarian cancer.
- Prostate-specific antigen: typically present with prostate malignancies and presents a non-invasive metric for disease monitoring.
- Carcinoembryonic antigen (CEA): associated with a broader number of cancers, such as breast, colorectal, gastric, lung, pancreatic, and ovarian. CEA requires attention to specificity determinations, as it can present in non-cancer cases.
There are several additional tumor markers illustrated below:
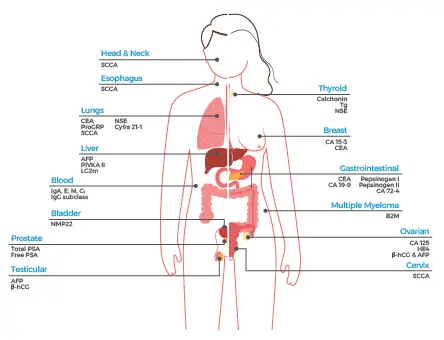
Circulating Tumor Cells Are Hard to Detect but Reduce Patient Burden
Tumor cells sometimes break away from the tumor and circulate in the bloodstream, allowing researchers to measure them as circulating tumor cells (CTCs). While detection can be onerous (the signal-to-noise ratio is ~one billion to one), CTCs are positive indicators of specific tumor types of solid cancers and can indicate the possibility of metastasis . However, following confirmed metastasis, CTCs may not be valid indicators of the state of the original tumor. These markers can inform treatment response and help reduce patient radiation exposure by reducing the need for repeated imaging assessments in clinical trials.
Most IO Biomarkers Require Composite Use
IO biomarkers can provide valuable data on a patient’s immune response to treatment and specification of which subpopulations would best benefit from immuno-oncology therapy. Based on previous studies, a single marker may be insufficient, and therefore, research points to the need for a “composite biomarker” made up of multiple markers meaningfully combined to inform the current disease state. Some examples include programmed death-ligand 1 expression on tumor cells, microsatellite instability, and tumor-infiltrating lymphocytes. The IO biomarker field is rapidly evolving—check out this white paper for the latest details regarding IO biomarkers.
Operational Considerations for Biomarker Use
Biomarkers are a key part of cancer research, but you must consider critical operational factors before implementing them into your early phase clinical trial. For instance, have you defined the role of the biomarker and ensured you selected one appropriate for the role? Have you thought about how to codify and quantify your biomarker readouts? How will your biomarkers be collected, and are the timelines feasible? Are there future uses for the biomarker that will ultimately warrant regulatory concerns?
Read the complete white paper, Biomarkers in Oncology Studies, where we cover:
- Various methods for biomarker collection
- Features of an ideal circulating biomarker
- Detailed operational considerations for successful implementation
- Turnaround time for use and analysis of biomarkers
- Associated costs and cost-reduction approaches…and more.
Worldwide Clinical Trials has exceptional oncology leaders ready to address the needs of your study; contact us today.


