By Michael Sullivan, Senior Research Fellow, Worldwide Clinical Trials
Small molecule biomarkers provide insight into metabolic pathways related to therapeutic treatment and can help explain the mechanism of action during drug development for an array of therapeutic areas.

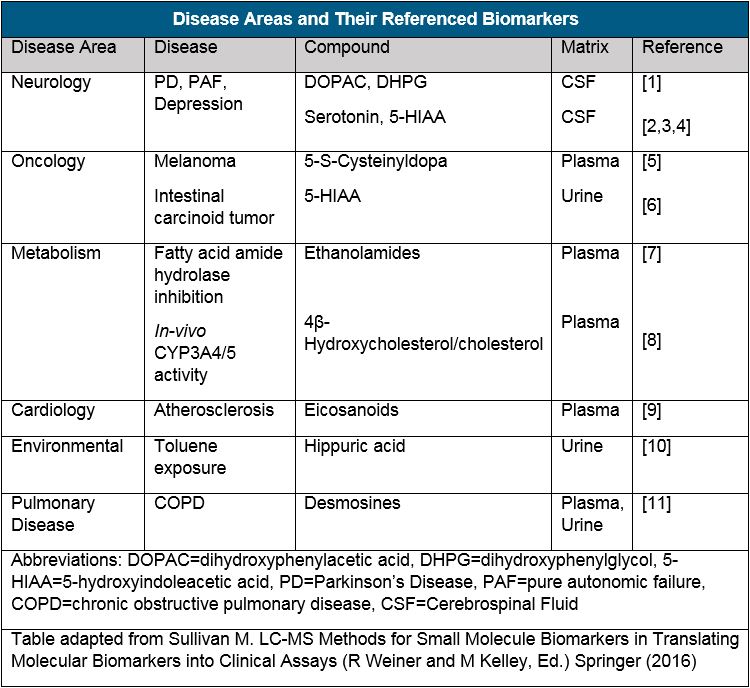
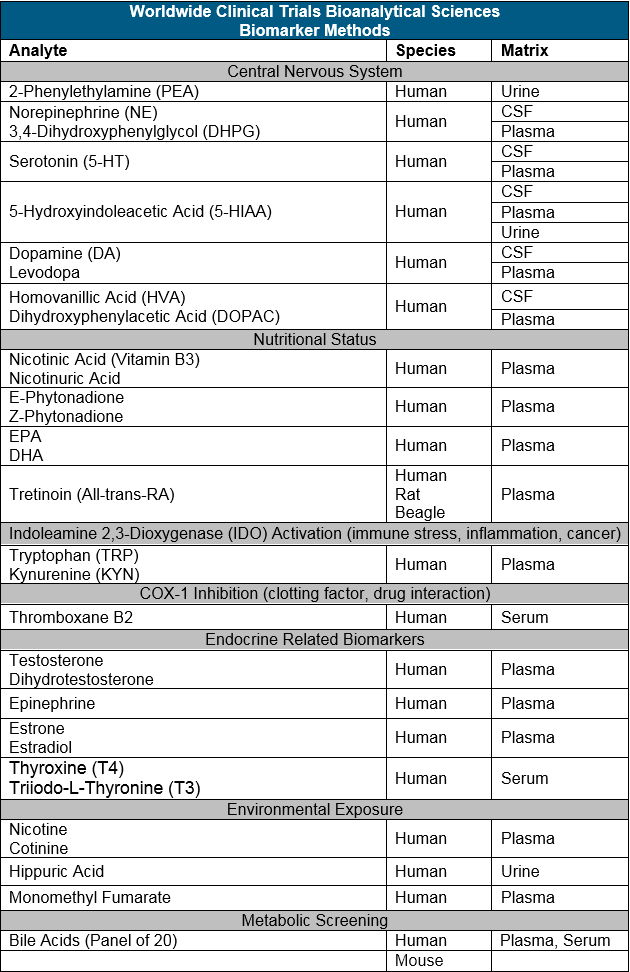
The research area of metabolomics is a good source of candidates for potential small molecule biomarkers, just as larger molecule biomarkers are found in the proteome. These small molecular weight metabolites and substrate are used as tools to provide insight into the biochemical pathways relating to many different disease areas (see table below).
Small molecule bioanalysis is usually performed using liquid chromatographic techniques coupled to mass spectrometric detection (LC-MS). This approach to biomarker analysis addresses the general challenges related to quantifying small molecules and provides low level quantitation, excellent selectivity, and fast separation from other small molecules. However, small molecule biomarkers are often endogenous to the control matrix that would otherwise be used for preparation of calibration samples, adding an additional challenge for quantification.
Pharmacokinetic and Biomarker Assay Development Share Similarities
Small molecule biomarker assay development is approached in a similar way to developing a small molecule pharmacokinetic (PK) assay. The advantages to this approach are:
- Results tend to be extremely accurate (compared to ligand binding/radio-immunoassays).
- Detection specificity is high due to mass selectivity (for LC-MS).
- Detection sensitivity is high due to the elimination of background noise.
Method performance for PK assays are held to a high standard through a method validation process outlined by regulatory agencies (FDA, EMA). Criteria defining accuracy and precision limits of results are the foundation of these method validation standards. Biomarker assays used for the purpose of collecting data for regulatory submissions are commonly held to these same high performance standards. These biomarker data are typically collected during late stages of drug development and help:
- Assess safety
- Assess efficacy
- Support dosing
- Support other patient treatment (drug interactions, food effects, contraindications)
Biomarker Assay Performance Criteria may Change to Support Internal Decisions
There are situations where small molecule biomarker assay performance requires less stringent performance criteria. This is usually at an early stage of drug development and the purpose of the biomarker data is usually for internal decision making regarding:
- Proof of concept for the mechanism of action
- Viability of a drug candidate
- Candidate screening
Most bioanalytical labs have procedures established for either approach. At Worldwide Clinical Trials there are standard operating procedures (SOPs) in place for method testing (including biomarker methods) to satisfy requirements from early to late stages of drug development.
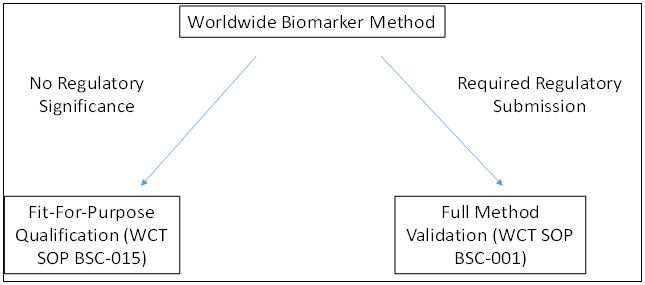
Under Fit-For-Purpose Qualifications, a lot of flexibility in assay performance is offered depending on the purpose of the data. In contrast, the SOP for Full Method Validation outlines what is needed for complete compliance with regulatory guidelines for accurate method performance.
Small molecule biomarker assay development is a good compliment to traditional PK bioanalysis. The tools/instruments are the same across the two areas and the assays perform to very tight standards. Bioanalytical assays that have been developed at Worldwide Clinical Trials that are useful for various drug development programs are listed below.
Learn More about our Bioanalytical Assay Development Methods
For more information about how our scientific team leverages metabolomics research in various therapeutic areas, or to learn more about biomarker and other small molecule bioanalytical methods available at Worldwide Clinical Trials, contact us here.
References:
- Goldstein DS, Holmes C, Sharabi Y, (2012) Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 135(6), 1900-1913.
- Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S, Eldadah BA, (2008) Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism and related disorders 14, 600-607.
- Hou C, Jia F, Liu Y, Li L, (2006) CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain research 1095, 154-158.
- Vincent S, Bieck PR, Garland EM, Loghin C, Bymaster FP, Black BK, Gonzales C, Potter WZ, Robertson D, (2004) Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation 109, 3202-3207.
- Martin G, Mansion F, Houbart V, Paquet P, Rorive A, Chiap P, Crommen J, Servais AC, Fillet M, (2011) Pre-study and in-study validation of a SPE-LC-MS-MS method for the determination of 5-S-cysteinyldopa, a melanoma biomarker, in human plasma. Talanta 84, 280-286.
- Perry H, Keevil B, (2008) Online extraction of 5-hydroxyindole acetic acid from urine for analysis by liquid chromatography-tandem mass spectrometry. Annals of clinical biochemistry 45(2), 149-152.
- Jian W, Edom R, Weng N, Zannikos P, Zhang Z, Wang H, (2010) Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibition in human plasma. Journal of chromatography B 878, 1687-1699.
- Xu Y, Yuan Y, Smith L, Edom R, Weng N, Mamidi R, Silva J, Evans DC, Lim HK, (2013) LC-ESI-MS/MS quantification of 4β-hydroxycholesterol and cholesterol in plasma samples of limited volume. Journal of pharmaceutical and biomedical analysis 85, 145-154.
- Rago B, Fu C, (2013) Development of a high-throughput ultra performance liquid chromatography-mass spectrometry assay to profile 18 eicosanoids as exploratory biomarkers for atherosclerotic diseases. Journal of chromatography B 936, 25-32.
- Penner N, Ramanathan R, Zgoda-Pols, J, Chowdhury S, (2010) Quantitative determination of hippuric and benzoic acids in urine by LC-MS/MS using surrogate standards. Journal of pharmaceutical and biomedical analysis 52, 534-543.
- Miliotis T, Lindberg C, Semb KF, van Geest M, Kjellstrom S, (2013) Quantitative high-performance liquid chromatography-tandem mass spectrometry method for the analysis of free desmosines in plasma and urine. Journal of chromatography A 1308, 73-78.