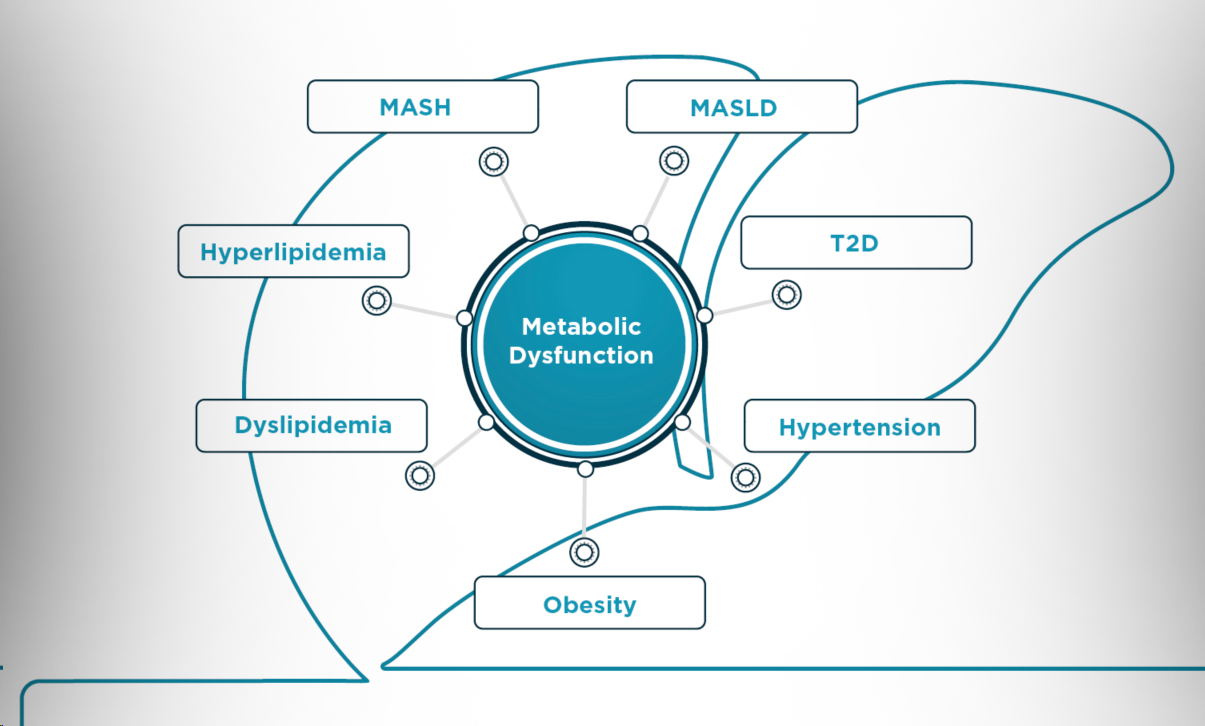
Advance the development of your therapeutic for metabolic dysfunction-associated steatohepatitis (MASH) or metabolic dysfunction-associated steatotic liver disease (MASLD) with an experienced team. We provide tailored, data-driven solutions to ensure the success of your trial. Learn more about our strategic clinical development services, scientific expertise, and strong relationships with KOLs and sites.