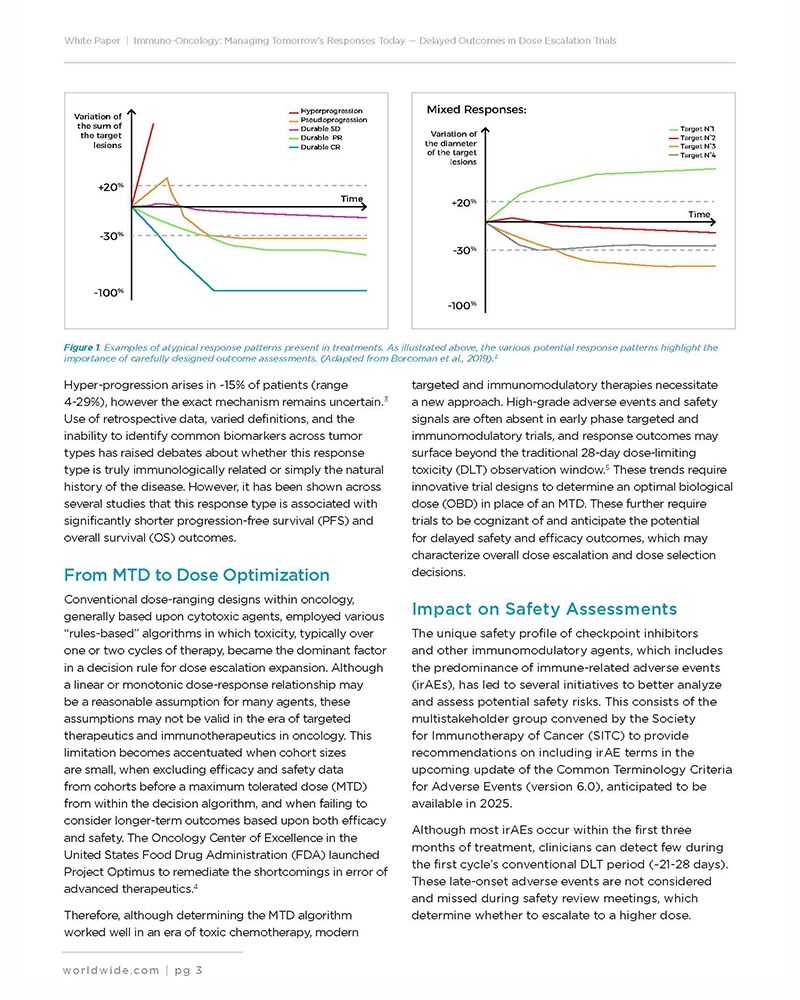
Immunotherapy has reinvented our perception of cancer treatment, yet the immune system’s complexity leaves mechanisms that are still not fully understood. This complexity can lead to side effects appearing weeks or months after treatment onset. Although current guidelines offer strategies for managing these delayed immune-related adverse events (irAEs), innovative clinical trial designs are needed to effectively identify and evaluate late-onset toxicities and delayed efficacy responses in early phase research.
In our white paper, you can learn more about:
- Atypical responses to immunotherapy and how they impact trial design
- Delayed event impacts on safety and efficacy assessments
- Statistical method decisions and influence on trial design
- Current guidelines and how to best position your immuno-oncology trial up for success