In 2017, the National Institute of Aging and Alzheimer’s Association (NIA-AA) proposed a common research framework to help clinical investigators establish a research agenda and evaluate the impact of various therapeutics in the Alzheimer’s disease (AD) continuum, and specifically to define and stage AD and facilitate research reporting.

To understand the implications for Alzheimer’s disease research, Talking Trials spoke with Worldwide’s Natalia Drosopoulou, Ph.D., Senior Director of Project Management in Neuroscience. This is Part 1 of a three-part series addressing the NIA-AA’s research framework and some of the more salient issues related to its implementation in clinical trials of AD therapies (1).
What is the purpose of this Alzheimer’s disease research framework, and how did it originate?
This research framework was revealed at the Alzheimer’s Association International (AAIC) conference that took place in London this July with an accompanying draft document posted on the Alzheimer’s Association’s website (2). The authors were quick to point out that this update to the 2014 International Work Group (IWG) diagnostic criteria (which requires the presence of cognitive symptoms plus biomarker evidence of AD pathophysiologic processes for a diagnosis of AD) and to the 2011 NIA-AA diagnostic criteria was meant to represent a research framework to be used in observational and randomized interventional trials and not to be viewed as general clinical diagnostic criteria for use in clinical practice (3).
This update was deemed necessary due to recent developments in conceptualizing AD as being on a continuum (rather than as three separate clinical constructs of preclinical, mild cognitive impairment, and dementia) occurring over long periods of time that can be accurately reflected wholly by biomarker progression commencing long before symptoms become apparent.
The proposed biomarkers, which reflect beta amyloid deposition, tau pathology, and neurodegeneration, are considered to be valid surrogates for pathophysiologic states associated with AD. This updated Alzheimer’s disease research framework allocates potential subjects into three biomarker classifications based on presence or absence of amyloid (A), tau (T), and neurodegenerative (N) biomarkers and then goes on to define clinical staging in terms of both syndromal categories and numeric schemes.
What is significant about the framework’s definition of Alzheimer’s disease?
The updated research framework pivots upon the understanding and acknowledgment that AD refers to an aggregate of pathophysiologic processes and therefore can be uniquely defined in vivo by biomarkers and, of course, by post mortem pathologic changes but conspicuously not by clinical symptomatology. This separation of clinical symptoms from the pathophysiological state represents a big departure from the antecedent NIA-AA criteria proposed in 1984 and updated in 2011(3, 4). This shift in thinking away from AD as a clinical-pathological construct and then as a clinical-biomarker construct (where biomarkers were used to support an AD diagnosis) represents the key feature to the updated research framework. This departure was buttressed by the awareness that dementia is not a disease but rather a syndrome composed of signs and symptoms that can be caused by multiple diseases, one of which is AD. In the current framework, AD is defined as a pathophysiologic construct that is uniquely and absolutely identified by biomarkers and only biomarkers that are specific to AD proteinopathies (e.g., Aβ and pathologic tau) should be considered as potential biomarkers of AD. Therefore, AD can be viewed exactingly and exclusively as a proteinopathy.
What guidance is offered for using biomarkers in Alzheimer’s disease research?
Drug developers have enjoyed a plethora of in vivo biomarkers to choose from in order to help define disease state and to track progression and treatment effects of AD drugs. Given the abundance of biomarkers, the updated research framework utilized an unbiased descriptive classification scheme to advocate for those categories of biomarkers purported to be the most useful in AD research endeavors. Specifically, the authors propose the “A/T/N” system in which seven of the most commonly investigated AD biomarkers were divided into three binary categories based on the nature of the pathophysiology that each ostensibly measures (5). In this scheme, the category letter labeled “A” refers to the value of beta amyloid biomarkers reflecting binding on amyloid Positron Emission Tomography (PET) or low CSF beta amyloid (Aβ42), which designates the presence of beta amyloid plaque and associated pathophysiologic processes. The category letter labeled “T” refers to tau biomarkers, such as elevated CSF phospho tau or tau PET binding, and reflects aggregated pathologic tau, and the category labeled letter “N” represents biomarkers of neurodegeneration or neuronal injury such as hypometabolism on [18F]-fluorodeoxyglucose–PET, atrophy as seen on structural MRI, or CSF total tau. Of note, there is both an imaging and a CSF biomarker in each of the three biomarker categories, making it possible to have complete characterization of Alzheimer’s biomarkers using a single modality, such as imaging or CSF alone.
How do these biomarkers help categorize Alzheimer’s disease?
Because a potential subject in an Alzheimer’s disease clinical trial can be either positive or negative on each of the three biomarker categories (ATN), there are eight possible biomarker profiles each corresponding to a label representing a range from normal biomarkers to AD pathophysiology to Alzheimer’s disease or non-AD pathophysiology (see Table 1).
Table 1 – Alzheimer’s Biomarker profiles and categories (2)
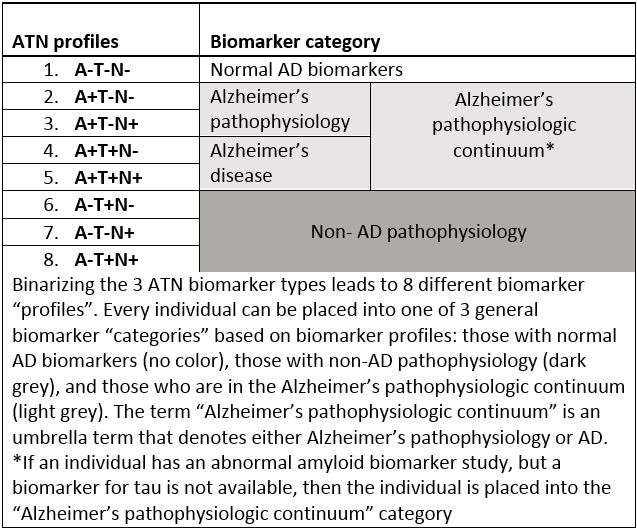
Using this NIA-AA research framework, a potential subject who has biomarker evidence of Aβ pathophysiology alone (amyloid PET or low CSF Aβ 42 or 42/40 ratio) but with a normal tau biomarker would be assigned the disease label “Alzheimer’s pathophysiology,” while the term “Alzheimer’s disease” would be used only if there was biomarker evidence of both Aβ and pathologic tau. Of note, in this model, “Alzheimer’s pathophysiology” and “Alzheimer’s disease” are not regarded as separate entities but rather earlier and later phases on the “Alzheimer’s pathophysiologic continuum,” and importantly, these labels have no regard to clinical symptoms (2).
Thank you for reading Part 1 of “Implications of NIA-AA Framework for Alzheimer’s Research.” Read Part 2 for a closer look at the NIA-AA diagnostic framework for using biomarkers in combination with clinical symptoms.
References
- Riordan HJ, Drosopoulou NE. Updated research criteria for clinical trials across the Alzheimer’s disease continuum. Journal for Clinical Studies 9:5. 2017 November.
- Jack CR Jr., Bennett DA, Blennow K, Carillo M, Dunn B, Elliott C, Haeberlein S, Holtzan D, Jagust W, Jessen F, Karlwash J, Liu E, Masliah E, Molineuvo JL, Montine T, Phelps C, Rankin K, Rowe C, Ryan L, Scheltens P, Siemers E, Siverberg N, Snyder H, Sperling R. 2018 NIA-AA research framework to investigate the Alzheimer’s disease continuum. Draft 7-18-17. http://alz.org/aaic/_downloads/draft-nia-aa-7-18-17.pdf.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia.2011 May;7(3):263-9.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939-44.
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. 2016 Aug 2;87(5):539-47.


