
Satish Kumar, MBB, Head of Process Improvement
Jayaprakash Kotha, MBBS, PhD, ASCP (SH), Vice President, Bioanalytical Laboratory
Continuous Innovation is a Cornerstone of Bioanalysis
Approximately 80% of drugs that begin the research process fail to reach approval. What is one contributing factor that sets the 20% that do apart from the rest? Rigorous procedures to ensure that drugs are effective and safe.
Regulatory bodies such as the FDA oversee clinical trials to ensure that studies’ design, conduction, analysis, and reporting are per established guidelines and laws. Bioanalysis during clinical development of a drug is an indispensable process where trials obtain critical data pertinent to pharmacokinetics (PK) and pharmacodynamics (PD) and as readouts that are crucial for assessing the safety and efficacy of the drugs. Bioanalysis to support drug development requires meticulous attention to detail across many clinic and laboratory disciplines. Therefore, processes that impact laboratory efficiency and data quality are essential and can confer time and cost savings and advance groundbreaking treatments for those in need. No matter how small, any lack of efficiency can snowball into costly delays in drug approval.
The impact of inefficient bioanalysis in drug development is accentuated in Phase I trials, as they require timely data gathering and quick decision-making, especially in dose escalation studies determining patient tolerability and safety. Any delays or missteps in bioanalysis during a Phase I trial can derail the trajectory of a promising drug. Successful bioanalytic (bioA) operations require a commitment to innovating change, which champions high-quality, efficient, and scalable opportunities for those conducting research. Partnering with the right CRO makes the difference in rapidly discovering novel, effective interventions. Here, we discuss essential components for successful innovation implementation drawing from WorldwideEdge™ our in-house team committed to constantly challenging the status quo, innovating our operations, and providing top-quality bioA for our customers and the industry.
Implementing Innovation
BioA processes require a laser focus on improvement, accuracy, and efficiency, further underscored by the growing pipeline of novel drug candidates. Success in this high-pressure environment requires optimized operations to rapidly support high volumes of Phase I novel candidate therapeutics. Meeting these demands requires a holistic approach that harmonizes people, processes, and technologies, making it imperative to employ a systematic strategy fueled by data that show clear improvements following novel method implementation.
Data-Informed Decision-Making
Procedural changes are often difficult to implement successfully, but with the appropriate strategy and integrated team, the rates for success increase significantly. The following broad-view approaches contribute to seamless implementation:
- Identify opportunities: Conduct regular audits to pinpoint inefficiencies, bottlenecks, and protocol challenges, ensuring alignment with strategic objectives.
- Select the right projects: Evaluate potential interventions for their broader impact and prioritize projects that address critical issues, optimize quality, or reduce costs.
- Earn team buy-in: Engage teams early and communicate the proposed changes’ benefits to build confidence and enthusiasm.
- Sustain momentum: Standardize successful improvements and establish mechanisms for ongoing reviews to maintain progress.
Our experts conduct regular audits to pinpoint inefficiencies, eliminate bottlenecks, and resolve protocol challenges. After identification, we evaluate the broader impact of a potential intervention on parallel processes, whether adverse or mutually beneficial. We then prioritize projects that align with strategic goals or resolve critical issues, particularly those with a measurable impact on quality, cost, or turnaround time. As a factor for implementation, our experts attend to every detail and ensure effectively allocated resources.
Anticipating & Proactively Addressing Challenges
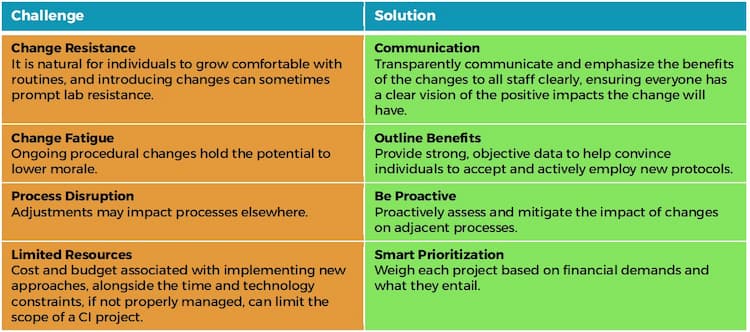
Change requires careful, ongoing attention and a proactive approach to addressing common challenges that impact full acceptance and implementation. However, challenges need not interfere with successful implementation when approached with appropriate, preemptive solutions (Table 1).
Table 1: Challenges & Proactive Solutions for Innovative Change Implementation.
Plan & Partner for Success
Successful innovation projects start with strong leadership. Top-down support is critical to frame any changes as opportunities rather than challenges, fostering lab-wide enthusiasm for implementation. Clear, measurable objectives created from data-driven decisions provide more motivation to adopt new procedures and impart cross-functional collaboration by bringing in teams across various functions, such as quality operations, to optimize success. True impact comes from cultivating a culture that celebrates change and innovation and empowers and rewards all staff for contributing to the efforts. This approach ensures that improvements are standardized and sustained to promote lasting improvements.
Transitioning your project from conception to experimentation is a pivotal moment, and many factors can determine if your drug will land in the 20% that succeed. Among these, selecting a research partner who prioritizes operational excellence is one of the most critical and controllable elements. At Worldwide Clinical Trials, WorldwideEdgeTM is our initiative to embrace efficiency, innovation, and teamwork. Focused on data-driven solutions, we ensure efficiencies at every stage of the process with the following:
Process standardization through enhanced operational efficiencies that maximize space and minimize parallel project interference or delays.
In-depth training ensures that every team member is confident and prepared to transition into new processes.
Eliminate waste by carefully reviewing all tasks and removing any that are superfluous, optimizing workflows and boosting overall productivity.
Enhanced error proofing with elevated monitoring ensures maximum risk reduction for all sample processing.
Centralized processes facilitate smarter resource allocation and site-wide understanding of changes. Our project-specific centralized dashboards provide visibility throughout the lab, across projects and teams, to drive ongoing, active engagement and ensure that all departments maintain an up-to-date understanding of current implementations.
Continuous feedback loops, like embedding Plan-Do-Check-Act (PDCA) cycles, foster iterative improvements and ensure sustainable outcomes.
Stakeholder engagement, including implementing a RACI (Responsible, Accountable, Consulted, Informed) framework to define roles, improve communication, and ensure alignment across teams.
Sustainability practices, such as incorporating eco-friendly practices into process design, implementing waste reduction, and energy-efficient operations, align with global sustainability goals.
Our approach has yielded tangible results:
- A 77% reduction in study delays caused by material availability
- A 38% increase in lab capacity
- A six-day reduction in the average cycle time for reference materials
- A 98% decrease in document search time
- Sample analysis right first time averaging 90% and above for last 9+ months
These metrics are a snapshot of the transformative impact of our initiatives. We remain committed to pushing the boundaries of efficiency and quality, ensuring that every partnership propels the drug development journey forward. When deciding upon a CRO to collaborate on your novel drug development journey, select one that shares your passion and commitment to your novel product.
Contact us to set up a time to meet with our bioA experts and discuss how our operations can serve your upcoming novel drug investigation.