Rich Bennett, Senior Director, Therapeutic Strategy Lead, Neuroscience and Peter Böhm, Senior Director Project Management, Neuroscience – Spain
Alzheimer’s disease (AD) continues to challenge the medical community due to its complex pathology and challenges, particularly with early detection, as patients should receive treatment as early as possible to alter the disease trajectory. This blog delves into the innovative approaches in AD therapeutics, highlighting the integration of fluid and imaging biomarkers that revolutionize clinical trial designs, potentially enhance the window of benefit of treatments, and allow for FDA accelerated approval. By examining the latest breakthroughs, including the FDA’s recent regulatory adaptations and the promising effects of monoclonal antibodies (mAbs), we provide a comprehensive overview of the current landscape and future directions in AD research. Together, we can contribute to breakthroughs in the field.
Alzheimer’s Disease Is a Global Concern
AD is a progressively debilitating neurodegenerative illness that presents clinical features of cognitive decline in the context of the accumulation of amyloid-beta plaques and tau protein tangles as the most prominent but not the only pathophysiological changes. Tau binds microtubules to stabilize them as they support healthy neurons. It becomes pathological when Tau detaches, aggregates, and forms neurofibrillary tangles (NFTs), a robust correlational measure of disease progression. Currently, more than 47 million individuals across the world are affected by dementia, and 60% to 80% of these diagnoses ultimately end up being classified as AD.
The financial burdens associated with AD drug research are considerable and reflective of the challenging nature of developing effective treatments for this condition. For example, despite extensive efforts in research and development, the success rate of clinical trials for treatments remains low, with approximately 99.6% failing. Despite these odds, the future holds promise, especially with recent breakthroughs in therapeutic options and the increasing potential for fluid and imaging biomarkers to serve as clinical endpoints.
Cause for Hope: Current Breakthroughs in Biomarkers & the Resurgence of Alzheimer’s Disease Clinical Trials
The FDA’s willingness to show regulatory flexibility and grant accelerated approval to two mAbs to treat AD in the last three years has been an example of how facilitated pathways intended to expedite a drug’s approval for a serious or life-threatening illness are becoming more commonplace. The FDA will grant accelerated approval when research shows potential therapeutic benefits over existing treatments. This is particularly the case when the drug can influence a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients when there remains some uncertainty about the drug’s overall clinical benefit.
Aducanumab was the first novel therapy approved for AD since 2003. This monoclonal antibody targeting amyloid beta plaques in the brain was the first drug to show reductions in this hallmark of AD. Despite the biomarker findings, uncertainties over the clinical benefit remained since the two Phase III trials. Only one met the primary endpoint of clinical decline according to the Clinical Dementia Rating-Sum of Boxes (CDR-SB) score at 18 months. Various interested stakeholders, such as the AD patient community, the press, and politicians, thrust this MAA review into the court of public debate because the advisory committee did not agree that the clinical benefit from one pivotal trial was sufficient for approval. As a note, this meeting in November 2020 did not discuss eligibility for accelerated approval. However, the drug was withdrawn from the market in 2024.
Fast forward to 2023, lecanemab, an amyloid-beta-directed monoclonal antibody, first received accelerated approval by the FDA in January and converted to traditional approval in June. This breakthrough therapy also benefitted from the FDA priority review, but the case differed from aducanumab. The CLARITY-AD Phase III study, with which Worldwide Clinical Trials supported, confirmed the clinical benefit observed in the sizeable Phase II study with consistency and statistical significance across all endpoints, including fluid and imaging biomarkers, which allowed for accelerated approval.
Appetite for Continued Progress
The consequence of seminal drug approval is an increased focus on development in this area. Lessons learned from study design and conduct and the establishment of more recent regulatory precedence contribute to the new state of the art in clinical development strategy. Based on our involvement in lecanemab, we gained a better grasp of the process and used these experiences to further optimize Alzheimer’s disease research strategy going forward. Overall, the continued unmet medical need and high burden on healthcare and society feed the patient, family, and political appetite for continued progress.
FDA Revised Early Alzheimer’s Disease Guidance
The FDA has issued a draft guidance document for Early Alzheimer’s Disease: Developing Drugs for Treatment, March 2024, for review comments by June 2024. The intention behind this draft guidance is to assist sponsors in the clinical development of drugs for the treatment of the stages of sporadic AD that occur before the onset of overt dementia. This draft guidance revises the previous draft guidance for the industry of the same name issued in February 2018.
General Considerations
Historically, AD clinical research has focused on overt (late-stage) AD, where subjects exhibited evidence of both cognitive changes and functional impairment. More recently, through the advancements in scientific understanding of fluid and imaging biomarkers, drugs are designed to target underlying AD pathology. This enables research to take place in significantly earlier stages of the disease, including pre-symptomatic/pre-clinical AD (stages 1-3), with the therapeutic intent of halting or reversing the pathophysiology.
Population
The guidance suggests using current consensus diagnostic criteria to confirm the biological presence of AD. It supports the use of biologically based diagnostic criteria grounded in a contemporary understanding of the pathophysiology and evolution of the disease. A requisite companion diagnostic could aid in assessing relevant biomarker levels at baseline. The study design should consider the patient’s disease stage at inclusion and the landmark primary outcome.
Endpoints & Observation Period
Historically, coprimary endpoints, including assessments of cognition and function, were used in overt AD stages 4-6 to ensure observation of cognitive changes with functional benefit and that observed changes in function were attributed to cognition and not another condition. This approach requires studies of two years or less. Establishing a meaningful treatment effect on cognition and function in early AD may require a more extended observation period. Many tools designed for overt AD would not be sensitive enough to detect subtle changes.
Therefore, the FDA may consider other approaches in early AD that make shorter trial durations permissive, such as cognition alone or surrogate endpoints. Time to Event (TTE) analysis of a clinically meaningful event might become a primary efficacy measure in early AD. Clinical trials showing an effect on a surrogate endpoint that is determined to be “reasonably likely to predict clinical benefit” can be the basis for accelerated approval, with post-approval trials required to verify and describe clinical benefit.
Surrogate Endpoints & Approval Pathways
Direct measures of clinical benefit or validated surrogate endpoints may support a traditional approval. The acceptability of a surrogate endpoint for use in a particular therapeutic development program for early AD may depend on the stage of disease, population enrolled in trials, therapeutic mechanism of action, and availability of current treatments. FDA has considered a reduction of the brain amyloid beta burden, as assessed by positron emission tomography, to be a surrogate endpoint that is reasonably likely to predict clinical benefit. It is, therefore, important to continue researching blood biomarkers that show promising results with less invasive and lower-cost procedures.
Accelerated approval pathways based on treatment effects on surrogate biomarkers such as amyloid beta plaques and NFTs are applicable beyond mAbs. They will increasingly form the foundational development strategy for other treatment modalities in AD, such as RNA technologies (siRNA). Different scientific approaches, modalities, and targets all have consequences on study design and participant experience. As shown below, there are 523 planned or ongoing AD clinical trials in 2024, with most trial starts focused on immunological therapeutics.
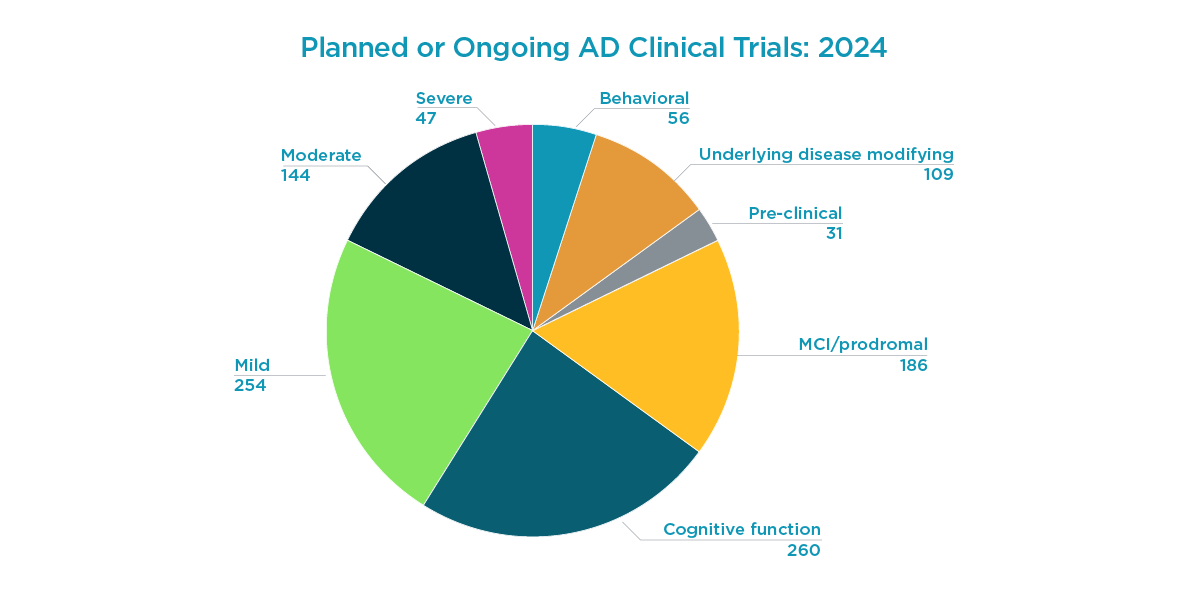
Trial starts in 2024 by drug class:
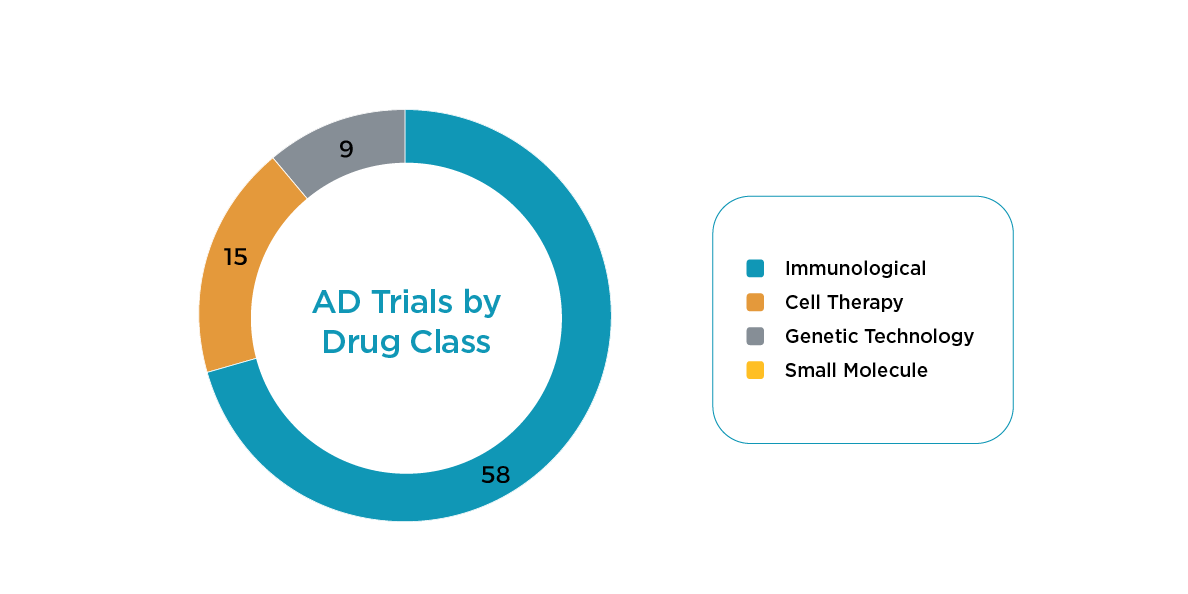
Factors Associated with Therapeutic Approach
- The immunological class of treatment uses an invasive route of administration and requires a titer for mAbs, which may exclude some otherwise eligible patients. This class has the most trials starting in 2024.
- Cellular therapy requires an invasive route of administration with cell harvesting for autologous approaches or significant use of immunosuppressants.
- Genetic (DNA/RNA, e.g., ASO tech) requires long-term (i.e., 5-year) immunogenicity testing, and as with the previous drug classes, it also involves an invasive administration route.
- Small molecule therapeutics have a higher frequency of dosing and consequential risk to IP compliance, creating additional hurdles to approval, with no current studies in 2024.
An Optimistic Future for AD Research
As we see hope with advancements in AD therapeutics, continuing the momentum gained from recent regulatory and scientific breakthroughs is crucial. Integrating surrogate biomarkers in clinical trials represents a pivotal shift towards more precise and early diagnostics, possibly leading to more effective treatments. Worldwide has a panel of Alzheimer’s research experts, a dedicated bioanalysis lab to validate and run high-volume biomarker assays, and a specialized Clinical Assessment Training and Surveillance Team that can support cognitive and functional tests. From regulatory nuances to intricacies of trial design and execution, our team is ready to help you; contact us for your next AD study!