For this two-part blog series, Talking Trials asked Worldwide’s Dr. Drosopoulou, author of the recent article, “A Methodology for Advancing the Selection of Biomarkers for Use in Psychiatric Clinical Trials,” published in the Journal for Clinical Studies, to weigh in on the use of biomarkers for clinical research.
Clinical trials using biomarkers in patient selection have higher overall success probabilities than clinical trials without biomarkers.1 That’s one of the many compelling results from a recent study of drug development success published in the journal Biostatistics. Wong and colleagues found clinical trials using biomarkers to stratify patients exhibit almost twice the overall probability of success compared to trials without biomarkers (10.3% vs. 5.5%).
Worldwide Clinical Trials has managed a growing number of clinical studies using biomarkers in the past several years. We propose here and in part 2, a methodology for advancing the selection of biomarkers for use in psychiatric clinical trials.2
With forethought, biomarkers can aid in clinical development of novel therapeutics
As psychiatric drug development advances toward more personalized medicine, the role of biomarkers has become integral to the investigation of the safety, tolerability, and efficacy of a range of psychiatric drugs. Biomarkers can aid in the development of novel therapeutic agents by supporting patient selection or enriching clinical trials with patients more likely to have a specific psychiatric illness (diagnostic and prognostic biomarkers); stratifying on inclusion criteria (predictive biomarkers); and by serving as an indicator of drug activity or even as a surrogate for clinical outcome or safety (pharmacodynamic biomarkers). The expanding use of biomarkers has raised many challenges in both the design and conduct of clinical trials and has underscored the need to establish a standard methodology that helps ensure valid data in support of these biomarkers.
Unfortunately, psychiatric drug developers currently lack sufficient supportive data, and often even the tools needed, to ensure the successful selection and implementation of psychiatric biomarkers in the drug development process and, as a result, too often choose to remain agnostic in terms of biomarkers. Many clinical developmental programs simply progress by merely applying a plethora of biomarkers over the course of development without much forethought as to the type of biomarkers that may be most useful for a particular phase of psychiatric clinical study, the evidence supporting such biomarkers, the patient subgroups most likely to benefit or to have safety concerns due to treatment, and even the linkage of these biomarkers to mechanism of action or various treatment outcomes.
Given this and the enormous burden of development and qualification of biomarkers in terms of both time and cost,3 the establishment of a standardized methodology to evaluate and select biomarkers for use in psychiatric clinical trial settings is warranted.
The current review will establish a process for the assessment and selection of neuroimaging biomarkers in schizophrenia clinical trials as an example of how to optimize biomarker utility in psychiatric clinical trials. Using this methodology, a relatively large and disparate set of data reflecting wide-ranging biomarker value in schizophrenia trials can be evaluated qualitatively in a very efficient manner according to a predetermined level of evidence. Then, only the biomarker(s) that have the highest level of qualitative evidence grading are evaluated using the more time consuming but rigorous quantitative analytic techniques such as meta-analyses. It is important to note that although neuroimaging in schizophrenia serves as an example of this methodology, this procedure can be applied to any set of biomarkers (blood based, CSF, electrophysiology, etc.) across numerous psychiatric indications, such as depression, anxiety, substance use disorders, and ADHD.
Neuroimaging biomarkers may be more sensitive to target engagement and response
Numerous biomarkers including, but not limited to, electrophysiological, eye movement, genetic, cerebral spinal fluid, and blood-based biomarkers have been purported to show varied utility across schizophrenia clinical trial settings. Many of these biomarkers have been investigated to predict therapeutic response to antipsychotic drugs and the risk for agranulocytosis or metabolic side effects of various antipsychotics.4 Of particular note, it has become apparent that as a class, neuroimaging biomarkers provide an important measure of brain structure and function (and in some cases even behavioral response) and may offer a more sensitive marker of target engagement and treatment response than many of the aforementioned biomarkers.4
Although much of the research focus on biomarkers in schizophrenia has centered around functional MRI (fMRI) and FDG and receptor occupancy positron emission tomography (FDG PET, roPET) in the drug development process, it is clear that even more modest imaging biomarkers of brain structure have been shown to reliably track treatment effects, as brain structure possibly reflects some level of neuroprotection or enhancement of neuroplasticity associated with treatment.4 Therefore, it was important to evaluate the role of all available neuroimaging modalities that had valid pre- and post-treatment data as a measure of treatment response – and not just those related to function or receptor occupancy. These modalities include structural MRI (sMRI), functional MRI (fMRI), FDG-PET, roPET, SPECT, and diffusion tensor imaging (DTI).
Qualitative review provides initial evaluation of biomarkers
The qualitative evaluation of these various neuroimaging modalities was undertaken with the intent of suggesting biomarkers that were worthy of examination in a more rigorous quantitative statistical review. Given the provision of qualitative grading evidence, which is then supported by more quantitative confirmation, it is reasonable to conclude that the biomarkers eventually selected represent those that are most likely to have utility in the psychiatric clinical trial setting by being most likely to predict or track treatment response.
Schizophrenia treatment clinical studies that included structural or functional imaging modalities, including sMRI, fMRI, FDG-PET, roPET, SPECT, and DTI, were identified, categorized, and evaluated systematically by a biomarker working group from the International Society for CNS Clinical Trials and Methodology (ISCTM).5 Articles published after 1995 from human clinical trials on the abovementioned imaging biomarkers were selected from various databases including PsycInfo, MEDLINE, PubMed, and Google Scholar. The scientific level of evidence available for each imaging modality was evaluated in terms of its indication for
- treatment efficacy;
- prognostic outcome data;
- longitudinal change in disease, brain, or behavior with a focus on pharmacological treatment response;
- interaction with pharmacological target;
- linkage to clinical outcome; or
- sensitivity to change with treatment.
A standardized information template was utilized during review of each article for relevant data abstraction and collection. Information was gathered on the type of imaging modality, clinical trial design, intervention, patient population characteristics, and major efficacy findings for image-based region of interest (ROI), as well as an overall tolerability assessment. A qualitative evaluation of each psychiatric study and biomarker utility was performed using a grading of evidence level as outlined by Altar et al.’s prototypical process for creating evidentiary standards for biomarkers and diagnostics.6 This evidence matrix was applied to the evaluation of articles on seven types of evidence including
- theory of biological plausibility,
- interaction with pharmacologic target,
- pharmacologic mechanistic response,
- linkage to clinical outcome of a disease,
- mathematic replication confirmation,
- analytic validation, and
- relative performance – with resultant assignment of graded weight of evidence ranging from least to most (grades D to A).6
For example, under the evidence type for “theory of biological plausibility” grades range from D (observed association only) to A (human evidence-based mathematical model of biology showing biomarker is on causal pathway). Likewise, for the evidence type related to “interaction with pharmacologic target,” the grades range from D (biomarker identifies target in in vitro binding) to A (biomarker identifies target in in vivo studies or from tissues in humans, with accepted truth standard), and for the evidence type “pharmacologic mechanistic response,” grades range from D (in vitro evidence that the drug affects the biomarker) to A (human evidence that multiple members of this drug class affect the biomarker and the effect is specific to this class/mechanism).6
Over 800 article abstracts fulfilled the predetermined set of criteria for a primary review with over 100 articles receiving a more detailed review for qualitative evaluation and grading. Evidence grading varied by imaging modality, ranging from A- for sMRI to D for DTI, with resultant grades provided in Table 1.5
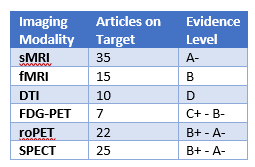
It was concluded from this qualitative analysis that the best imaging modality to move forward to the more rigorous quantitative analyses was sMRI, which was the only modality to receive an A- rating according to Altar criteria.6 Additionally, this qualitative review concluded that consistent methods for imaging acquisition and analysis in each imaging modality are warranted, that different imaging modalities may be best utilized at various phases of psychiatric clinical development and schizophrenia disease course, and that matching the imaging modality to a specific research question should result in better utilization of imaging biomarkers that are more sensitive to treatment effects in a clinical trial setting.5
Learn more about our expertise in psychiatric clinical trials
In part 2 of “A Methodology for Advancing the Selection of Biomarkers for Use in Psychiatric Clinical Trials”, we discuss the quantitative analysis of these results. To get in touch with Natalia to discuss biomarkers in neuroscience clinical trials, leave a question on her Expert page.
References
- Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 31 January 2018.
- Riordan H, Cutler N. A methodology for advancing the selection of biomarkers for use in psychiatric clinical trials. Journal for Clinical Studies. January 2018; 10(1).
- Riordan H. FDA’s new guidance on biomarker and patient reported outcome qualification. Journal for Clinical Studies. 2011;3(1):12-13.
- Murck H, Laughren T, Lamers F, Picard R, Walther S, Goff D, Sainati S. Taking personalized medicine seriously: Biomarker approaches in phase IIb/III studies in major depression and schizophrenia. Innov Clin Neurosci. 2015 Mar-Apr;12(3-4):26S-40S.
- Chen N, De Santi D, Fu D, van Kammen D, Moore C. van Erp T, Riordan H, Potkin S. A qualitative evaluation on evidence level for selective imaging biomarkers in schizophrenia clinical trials. Abstract presented to the International Society for CNS Clinical Trials and Methodology meeting; Feb 21-23, 2012; Washington DC.
- Altar CA, Amakey D, Bounos D, et al. A prototypical process for creating evidentiary standards for biomarkers and diagnostics. Clinical Pharmacology and Therapeutics. 2008;83(2):368-371.


