
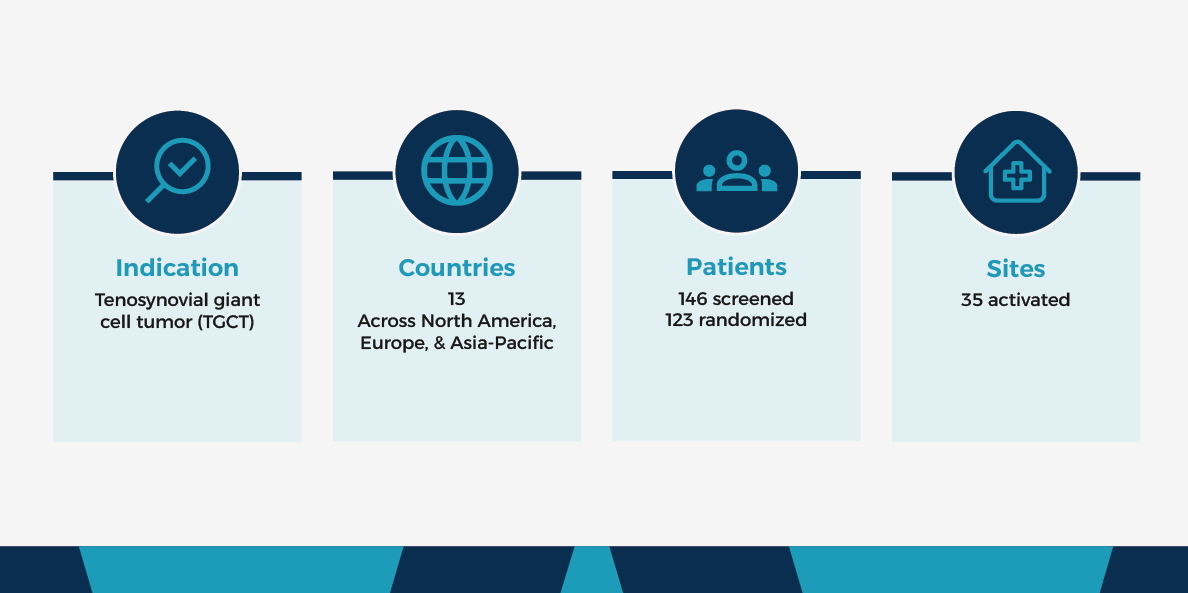
When a small biotech approached Worldwide more than nine years ago for a Phase I/II advanced malignancy study, this partnership resulted in an almost decade-long mutually beneficial collaboration. In 2023, when the sponsor’s tenosynovial giant cell tumor (TGCT) faced challenges, Worldwide’s team stepped in to foster increased collaboration and mitigate study roadblocks. Due to our team’s timely and efficient efforts, the TGCT study expects FDA and EMA approval, and Worldwide strengthened our relationship with the small biotech.
Optimizing Patient Recruitment
Initially, the study faced difficulties with patient recruitment in the U.S., as many patients were taking pexidartinib. This FDA-approved selective tyrosine kinase inhibitor is frequently prescribed for TGCT when surgery is not a viable option; however, its use rendered those prescribed ineligible for the clinical trial.
Despite initial challenges and a slight delay due to a protocol amendment, the study finished recruitment nearly nine months early due to our Data Coordinating Center’s collaborative and close relationships with sites. To help the sites once recruitment concluded so quickly, our team brought in additional SWAT Clinical Research Associate (CRA) support to stay on top of source data verification (SDV).
This supplementary support ensured each data cut-off and database lock went smoothly, as well as two FDA inspections in France and the Netherlands, with no citations. By concluding recruitment so early, the study did not incur additional costs and effort of opening sites in Latin America, which had been the original intention for its third cohort of patients.
Identifying Partnership Solutions
When collaborating on the TGCT study, the CRAs monitored SDV rigorously, ensuring a thorough review at each site, taking into account how difficult the site was and how efficient the CRAs were, the number of queries pending reply, and other relevant factors. We also tracked and followed up with many potential patients regarding their expected visit dates to the clinic to sign up for screening, which kept the sites engaged and focused on recruitment.
An added solution that significantly enhanced trust and transparency in the sponsor relationship was conducting a regular partnership survey. Through developing the questions in collaboration and completing answers independently, both sides were able to discuss the responses, hear constructive feedback from each other on what was occurring, and get invaluable information to propel the partnership in the right direction. The team further focused on a greater amount of face-to-face interactions, from in-person meetings to online check-ins with cameras on, to increase engagement and demonstrate active listening.
Strategic Partnership Milestones

Since our first collaboration, we’ve achieved two FDA and one EU approval, along with an additional approval expected in 2025. Supported by our industry-leading low turnover rates and responsive and engaged sponsors, this biotech is a proven example of how Worldwide builds and maintains strategic partnerships.
Get Dedicated Support from Worldwide
For your next clinical trial, reach out to our team at Worldwide to find out how we can accelerate your drug development efforts by aligning with your team’s culture to drive high performance and retention. While issues are inevitable in a study, work with a CRO that proactively addresses challenges as they arise to ensure your program stays on track. Contact us today to learn more about our personalized and therapeutically-focused approach.