
On January 12, 2004 when Queen Mary 2 set sail on her maiden voyage from Southampton, England, to Fort Lauderdale, Florida, in the United States, carrying 2,620 passengers, she was both the longest (345 meters – equivalent to 36 London double-decker buses) and largest (148,528 gross tonnage) passenger ship ever built. Whilst she no longer holds these distinctions, she remains the largest ocean liner ever constructed.

Impressive though the statistics are, if we pause for a moment to consider the nature and diversity of the individual systems needed to deliver a large, multinational cardiovascular outcome trial (CVOT), it’s not long before similarities between a cruise liner as sophisticated and complex as the Queen Mary 2 and the intricate, interconnected ecosystem of eClinical technologies deployed to support large and complex studies such as CVOTs become apparent.
For example, the role of the multitude of readouts and controls on the ship’s bridge are analogous to a study’s clinical trial management system (CTMS), which provides overall progress and management information to those steering the study, and risk-based monitoring (RBM) system, which directs the crew’s efforts to the most critical tasks.
The ship’s lookout is equivalent to the drug safety system, whilst the engine room driving the clinical study forward is powered by the clinical data management (CDM) and interactive response technology (IRT) systems.
Indeed, the more we look at what it takes to deliver a truly successful cruise, the more it starts to resemble a clinical study. For example, the typical tea consumption on a Queen Mary cruise would fill an Olympic-size swimming pool, whilst annual wine sales would be in the region of 230,000 bottles! I think the similarities are plain for all to see!
The key point is that piloting the Queen Mary 2 requires that the vast array of systems and subsystems, linked by miles of intricate wiring, work seamlessly together. Each crew member needs to be able to see the information critical to their role when they need it, enabling them to apply their years of training and knowledge to give clients the highest-quality experience they possibly can. When it all works, the technology itself becomes almost invisible – the ship seeming to glide effortlessly across the world’s oceans.
For Cardiovascular Outcome Trials, CROs Run a Fleet of Ships in Need of Regular Upgrades
As a CRO, we must consider a further level of complexity; we are running not just one but an entire fleet of ships, each subtly different in design and each heading for a different destination. What’s more, each time we head out on a voyage, some of the ship’s systems will have been upgraded or even replaced entirely. When we are considering technologies in clinical trials, change is truly the only constant.
And therein lies the greatest challenge – throughout each voyage the ship is being constantly buffeted by the unpredictable winds of increasing intricacy, diversity, and volumes of clinical data we need to collect and manage and risks being pushed off course by the swirling currents of a continuously evolving technology landscape.
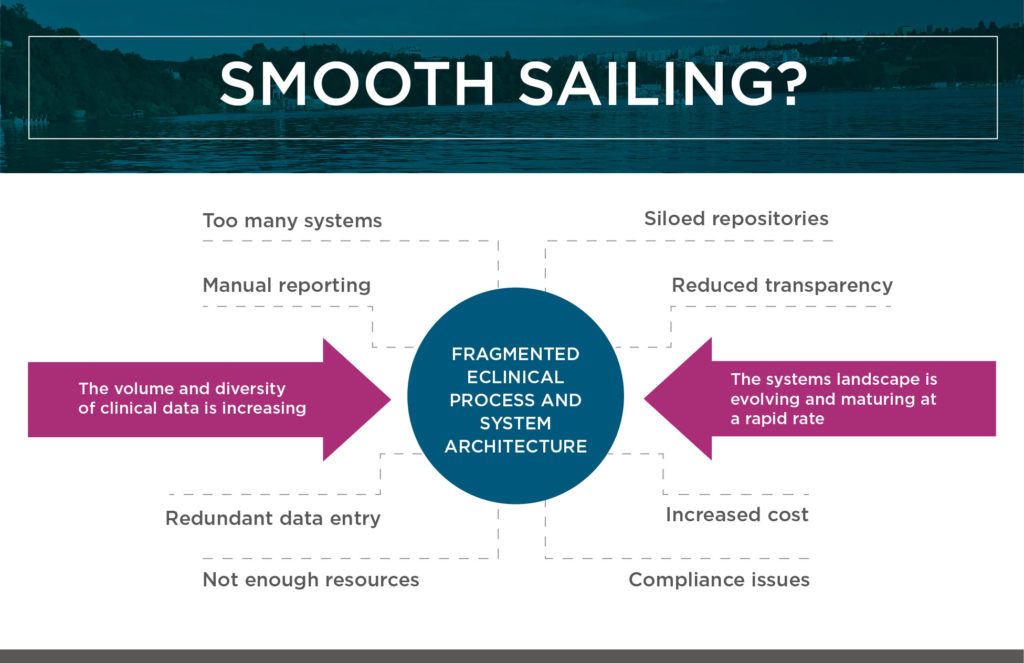
The result can all too easily be a fragmented and outdated ecosystem of eClinical technologies, which makes it hard (or even impossible) to maintain control, ensure compliance, measure performance, and maximize efficiencies. (See the figure titled “Smooth Sailing?” above.)
In my next blog in this series, we will look in a little more detail at some of the key trends driving technological change in the eClinical landscape and reshaping the future of cardiovascular outcome trials.
The Uncommon CRO for Your Cardiovascular Outcome Trial
If you’re looking to partner with a global CRO capable of executing full clinical research services for your next cardiovascular outcome trial, get in touch with the Worldwide experts. To learn more, check out our experience in cardiovascular clinical research.
About Chris Standley: With a background encompassing fields as diverse as invertebrate neurobiology and computer modelling, Chris first became involved in the technology side of clinical trials almost 20 years ago when he started developing software for data management in large cardiovascular outcome studies. Since then, he’s participated in a wide range of eClinical technology projects and is now responsible for Worldwide’s Vendor Governance and the eClinical Technology Roadmap.



